
MICROSCOPY AND
MICROANALYSIS
Expert scientists, one-of-a-kind equipment and industry connections
We are home to advanced microscopy, microanalysis, spectroscopy and imaging equipment.
These instruments are funded by government grants, Microscopy Australia and the Australian National Fabrication Facility (ANFF)—which means that they're available for use by scientists around Australia and the world. Our facility is led by a multi-disciplinary team of expert chemists, biologists, physicists and engineers undertaking novel research and providing service to industry.
Are you a researcher or scientist wanting to use our facility? Perhaps you have a business needing our people to improve your products or services? We believe in the power of working together to solve complex problems and find creative solutions, so get in touch.
OUR SERVICES
Cutting-edge technology
Our equipment and facilities are cutting-edge and often custom built. Many are one-of-a-kind in Australia—or even the world. They are used by researchers working across materials science, nanotechnology and nuclear chemistry on exciting projects like replacing toxic mining materials with rock-eating bacteria or creating solar cells that could one day power buildings.
Industry solutions
We work with businesses—from start-ups to multinational companies. Our researchers have improved products and processes across many industries, including defence, food and agriculture, manufacturing, maritime, and technology. We use our advanced optical and biomedical imaging and materials characterisation equipment to bring creative solutions to industry. Our past projects cover everything from looking the surface properties of materials to prevent corrosion, to correcting defects in 3D printing technology. Learn more →
Sharing our resources
We believe in the power of collaboration. By working together, we can solve complex issues and find innovative solutions. Our researchers are part of nationwide networks, with access to the Australian Synchrotron, the Australian Nuclear Science and Technology Organisation and more. We are creative big picture thinkers and detail-orientated problem solvers. What we all share is a deep love of science. But we know that science needs to work hand-in-hand with the real-world of politics, economics and social factors.
Cutting-edge technology
Our equipment and facilities are cutting-edge and often custom built. Many are one-of-a-kind in Australia—or even the world. They are used by researchers working across materials science, nanotechnology and nuclear chemistry on exciting projects like replacing toxic mining materials with rock-eating bacteria or creating solar cells that could one day power buildings.
Industry solutions
We work with businesses—from start-ups to multinational companies. Our researchers have improved products and processes across many industries, including defence, food and agriculture, manufacturing, maritime, and technology. We use our advanced optical and biomedical imaging and materials characterisation equipment to bring creative solutions to industry. Our past projects cover everything from looking the surface properties of materials to prevent corrosion, to correcting defects in 3D printing technology.
Sharing our resources
We believe in the power of collaboration. By working together, we can solve complex issues and find innovative solutions. Our researchers are part of nationwide networks, with access to the Australian Synchrotron, the Australian Nuclear Science and Technology Organisation and more. We are creative big picture thinkers and detail-orientated problem solvers. What we all share is a deep love of science. But we know that science needs to work hand-in-hand with the real-world of politics, economics and social factors.
Flinders Microscopy and Microanalysis has been formed to serve the nanoscale science, characterisation and imaging needs of our researchers, government and industry in South Australia and beyond.
Professor Sarah Harmer
Director, Flinders Microscopy and Microanalysis
OUR EQUIPMENT
We believe in the importance of sharing. We want to collaborate with researchers and scientists around the world by offering access to our innovative technologies. If you have a project that needs our equipment, we want to help. Our equipment falls into two categories: optical microscopy and materials characterisation.
Light microscopy and sample preparation
Our light microscopy and sample preparation capabilities provide a range of imaging solutions, ideal for imaging cells and other biological samples, such as live or fixed cells and tissue sections. We provide support and advice spanning custom sample preparation (including sample preparation equipment, validated reagents and consumables) through to sophisticated 3D image data analysis. Access to equipment for sample preparation and labelling or staining is also available.
For enquiries relating to our optical and biomedical imaging capabilities, please contact microscopy@flinders.edu.au.
The Zeiss LSM 880 Fast Airyscan laser scanning confocal microscope is capable of near-super resolution imaging of commonly used fluorescence dyes on live cells—as well as fixed samples—without the need for special sample preparation techniques or software deconvolution. This inverted microscope is equipped with environmental control and an autofocus device for short and long term live cell imaging and fast spectral data acquisition with on-the-fly spectral unmixing, enabling FRET and FRAP to be assessed in real-time and fluorophores/autofluorescence with overlapping emission spectra to be discerned. This instrument features 405, 458, 488, 514, 561 and 633 nm laser lines; a range of dry, water-immersion and oil-immersion objectives; versatility of imaging speeds; very high sensitivity and signal to noise ratios; 2 flanking PMTs, a single-channel GaAsP detector and a transmitted light detector and; DIC optics; set on an anti-vibration table.
Training requirements
In person training to be completed with relevant staff.
Contact people
The VS200 slide scanner is configured for automated imaging of up to 126 slides via fluorescence, brightfield, phase contrast, darkfield and/or polarization microscopy. Various high quality objectives (2x, 4x, 10x, 20x, 40x, 40x oil), fluorescence filters (DAPI, FITC, TRITC, Cy5, Cy7, CFP, YFP, mCherry) and cameras (monochrome and colour CMOS cameras) can be employed. High performance automated ‘count and measure’ and ‘deep learning’ software modules are available and separate data analysis and data storage PC workstations support the VS200 image acquisition system.
Training requirements
In person training to be completed with relevant staff.
Contact people
The Incucyte SX5 Live-Cell Analysis Instrument is contained within a large (230L) cell culture incubator (37°C, 5%CO2) and enables long-term live cell imaging using high definition phase contrast and up to 3 fluorescence channels in a single experiment (green /orange / near IR) in combination with 4x, 10x or 20x objectives. The system features 3 interchangeable trays that can be used with a large variety of cell culture vessels and up to 6 microplates in parallel. A large number of purpose-built software modules (and relevant accessories) are available for various applications in cell health and proliferation, cell function, 3D cell models, cell movement and morphology (Cell Migration/Invasion, Chemotaxis, Spheroid, Organoid, Cell-by-Cell, Neurotrack, ATP Analysis and Angiogenesis software modules are available.
Training requirements
In person training to be completed with relevant staff.
Contact people
The Cytation 5 combines automated fluorescence and brightfield microscopy and various microplate detection read-outs (fluorescence, luminescence, UV/Vis absorbance, fluorescence polarization and time resolved fluorescence). Imaging can be performed using various plates (including 96- and 384-well plates), slides (including various chamber slides), objectives (4x, 10x, 20x and 40x) and fluorescence filters (DAPI, GFP, Texas Red and Cy5). The dual reagent injector module is also available for use in flash-type luminescence assays and other controlled reagent delivery into microplate wells. Environmental control (temperature and CO2) enables performance of long-term live cell imaging experiments.
Training requirements
In person training to be completed with relevant staff.
Contact people
The IX83 widefield fluorescence microscope has been customized for sensitive imaging of fluorescently labelled samples. The system features an iXon Life 888 EMCCD camera (Andor) that is configured for ‘SRRF-Stream+’ super-resolution imaging and enables sensitive imaging with fast frame rates and/or low-light illumination of samples. Illumination is provided by a pE-800 LED light engine (CoolLED) equipped with corresponding fluorescent filters (DAPI, CFP, FITC, YFP, TRITC, mCherry and Cy5). A range of objectives are available (10x, 20x, 40x, 60x, 100x) and live cell imaging (37°C, 5%CO2) is supported using a stage-top incubator (Okolab) and an IX3-ZDC2 motorized Z drift compensator.
Training requirements
In person training to be completed with relevant staff.
Contact people
We have a range of compound and inverted epifluorescence microscopes equipped for camera-based digital image acquisition of slide-mounted samples as well as live cell or organoid cultures in a variety of culture dish types. These are set up to image all common blue, green, red and far-red emitting fluorophores with negligible cross-excitation or emission bleed through. These epifluorescence microscopes enable inexpensive screening and imaging of samples with minimum training required.
Contact people
We have brightfield compound and stereo microscopes equipped for full colour camera-based digital image acquisition, enabling inexpensive imaging of a variety of samples with minimum training required.
Contact people
Comprehensive sample preparation expertise and equipment is available to cater for custom preparation of live cells as well as fresh, frozen and fixed tissue samples. We have automated embedding equipment (Leica HistoCore PEARL) and a range of microtomes, sledge microtomes, ultramicrotomes, vibratomes and cryostats available. We can also provide a range of reagents and consumables, including validated secondary antibodies (enzyme and fluorophore conjugates), routine and specialist colourimetric stains, slides, coverslips and coverglass-bottom chambers/dishes. Researchers can opt for sample preparation to be provided or to be trained to access our sample preparation facilities.
Contact people
Materials characterisation
Our unique suite of instruments provides high precision surface chemical and physical characterisation and excellent spatial resolution. We are enabling innovations in nanotechnology, characterisation, defence, health, earth and environmental systems, mining, and advanced manufacturing, and we have a crucial role in advancing research and Australian business in collaboration with national facilities.
To find out more about our materials characterisation capabilities, please contact microscopy@flinders.edu.au.
Scanning Electron Microscopy (SEM) uses a beam of electrons to image to a much higher resolution than is possible with an optical microscope. High resolution SEM of samples can be combined with elemental mapping using Energy Dispersive X-ray spectroscopy (EDX). An Electron Backscatter Diffraction (EBSD) detector is also installed allowing measurements of grain orientation and boundaries in crystalline samples.
Specifications
FEI Inspect F50
Training requirements
In person training to be completed with relevant staff, complemented by online modules through MyScope.
Contact people
The Scanning Auger Nanoprobe is one of only two in Australia and is able to map chemical information across a surface. This instrument combines microscopy with the ability to determine elemental composition, resulting the analysis of surface chemistry with a spatial resolution of 10 nanometres.
Specifications
PHI-710 AES
Training requirements
In person training to be completed with relevant staff.
Contact people
The large-volume micro-CT scanner will allow 3D scanning of large and heavy samples. This includes whole machine parts, human and animal limbs or segments, biomaterials, prosthesis devices, large animals and vertebrates, fossils and plant root systems for research and industrial applications. This will also allow experimental testing rigs, such as mechanical stages or environmental chambers, to be placed inside the scanner (in situ testing), for testing samples while scanning.
Specifications
Nikon XT H 225ST CT Scanner
Contact person
Two sputter coaters are available for use. A single target unit is dedicated to sputtering samples for scanning electron microscopy (SEM) analysis. There is also a dual target sputtering system that is fully automatic, ideally suited for multilayer thin film applications. A range of metals including gold, silver, platinum, titanium and chromium are available.
Training requirements
In person training to be completed with relevant staff.
Contact person
The instrument allows for applying four different electron spectroscopy techniques; each technique can be applied independently. Metastable Induced Electron Spectroscopy (MIES) is a technique that is exclusively surface sensitive. This technique probes the valence orbitals of only the outermost layer of atoms, allowing for the determination of molecule orientation. This technique can be paired with three more electron spectroscopy techniques probing occupied and occupied states at various depth: Ultraviolet Photoelectron Spectroscopy (UPS), Inverse Photemission Spectroscopy (IPES) and X-ray Photoelectron Spectroscopy (XPS).
Specifications
Custom built in collaboration with SPECS.
Training requirements
In person training to be completed with relevant staff.
Contact people
Funded by Microscopy Australia and the Australian National Fabrication Facility
Neutral Impact Collision Ion Scattering Spectroscopy (NICISS) allows for depth profiling of a sample. This technique gives an elemental concentration profile to a depth of 40 nanometres, with a depth resolution close to 0.3 nm near the surface. NICISS can be applied to solid samples, polymers and liquids.
Specifications
Custom built in collaboration with SPECS.
Training requirements
In person training to be completed with relevant staff.
Contact people
Funded by the Australian National Fabrication Facility
Atomic Force Microscopy (AFM) is used to gain topographic information on a sample. Our AFM facilities are also able to map sample conductivity on the nanoscale, characterise stiffness and adhesion in air and fluid environments, and monitor dynamic changes in surfaces with our fast-scanning AFM, which is capable of acquiring images over 100 times faster than a conventional AFM.
Specifications
Multimode 8 AFM with Nanoscope V controller
Dimension FastScan AFM with Nanoscope V controller
Training requirements
In person training to be completed with relevant staff, complemented by online modules through MyScope, instructional training videos and tests.
Contact person
Our labs are equipped with two confocal Raman microscopes capable of acquiring single Raman spectra—and also confocal Raman—imaging. The maximum possible lateral resolution for confocal Raman images at the laser excitation wavelength of 532 nm is approximately 360 nm. Several excitation wavelengths are available, including 532, 632 and 785 nm. A selection of gratings is also available from 600 grooves/mm up to 2400 grooves/mm.
Tip Enhanced Raman Spectroscopy (TERS) combines a surface probe with Raman spectroscopy, allowing for chemical mapping of a surface down to a few tens of nanometres. The laser excitation wavelength is 532 nm.
Specifications
WITec alpha300R confocal Raman microscope
XplorRA Horiba Scientific confocal Raman microscope
Training requirements
In person training to be completed with relevant staff.
Contact person
For the Witec alpha300R confocal Raman microscope please contact Dr Chris Gibson
For the XplorRA Horiba Scientific confocal Raman microscope and TERS please contact Dr. Jason Gascooke
X-ray Diffraction (XRD) is a characterization technique used for examining the crystal structure of all materials. The instrument is a Bragg-Brentano geometry X-ray Diffractometer (XRD) with a cobalt X-ray source. It is ideal for qualitative phase identification, quantitative phase analysis and the determination of crystal structure. The cobalt source allows this instrument to accurately analyse high iron content samples. In addition to this, the instrument is equipped with a capillary stage for the measurement of a very small amount of sample. It is also useful for spinning samples that have issues due to high absorbances or texture effects. Data analysis capabilities for XRD include the use of the ICDD PDF-2 Database, DIFFRAC. EVA Software for phase identification as well as the Topas software package (Rietveld refinement method) for crystal structure determination and quantitative phase analysis.
Specifications
Bruker D8 Advance Eco
Training requirements
In person training to be completed with relevant staff.
Contact person
How to book your training
All users of our equipment must be trained by our staff. Before booking, please get in touch about your requirements and arrange a time to complete the relevant training. Once you are an authorised and trained user, you may go ahead and book the equipment using the links.
INDUSTRY SOLUTIONS
Flinders Microscopy and Microanalysis offers a range of characterisation services to businesses, from small start-ups to multinational companies.
We are a multidisciplinary team.
We are experts in biology, chemistry, physics, nanotechnology, geology, materials science and engineering. If you have a research or development project you want to pursue, we want to help you foster innovation. We will work with you to customise our testing and consultancy services to your needs. You can draw on our research and technical expertise, access our microscopy facilities and equipment, and even access a whole new range of government and research grants.
Let us help you develop new products or processes.
We can improve your existing products and processes—or help you create new ones. We are able to provide high resolution imaging and analysis, resulting in firm outcomes and recommendations. Our past projects have looked at the surface properties of materials to prevent corrosion and corrected defects in 3D printing technology. Typically we work with polymers, particle coatings and films, alloys and metals, plastics, and many others. We find and resolve defects and investigate ways to improve what's already working.
Working with industry and developing new techniques
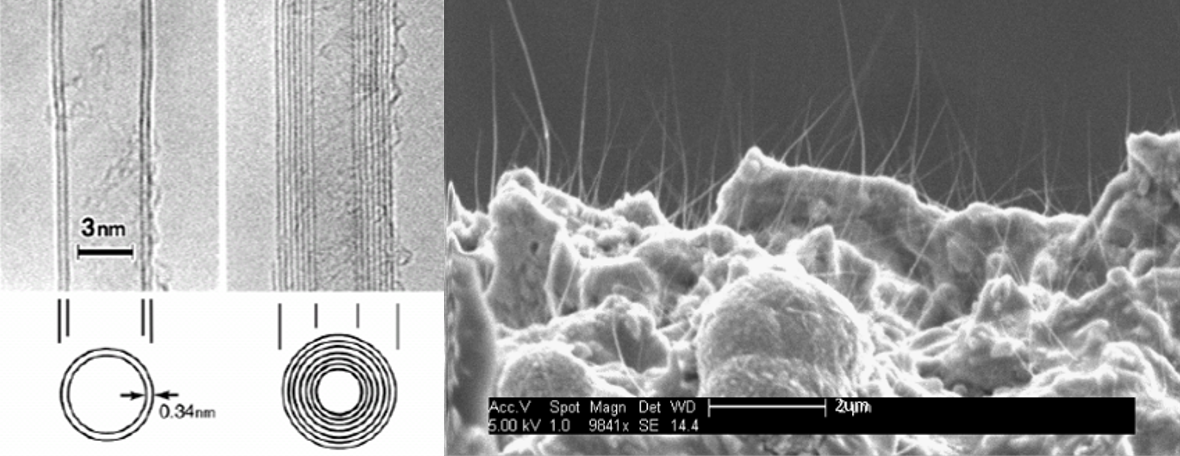
Micro-X Ltd designs, develops, and manufactures a range of innovative, ultra-lightweight, mobile x-ray imaging systems for medical and security applications. The competitive advantage of these miniaturised products stems from a new technology of electronic x-ray tube using carbon nano-tube (CNT) field emission devices, enabling the miniaturisation of a number of x-ray applications relevant to large global markets.
Demands for high throughput and resolution medical and inspection techniques such as radiotherapy, computed tomography and tomosynthesis, security inspection and high throughput manufacturing has resulted in a considerable level of interest in x-ray sources capable of meeting these requirements.
Micro-X has a contract from the Australian Department of Defence to develop and demonstrate the technology of an ultra-lightweight, digital mobile x-ray which is optimised for use in military deployed medical facilities. The product also has potential applications in humanitarian aid and disaster relief. The third product in Micro-X’s development pipeline is a mobile backscatter imaging device in the form of a miniature x-ray system allowing for stand-off imaging of improvised explosive devices.
Collaboration with the Flinders Institute for Nanoscale Science & Technology, with help from a business innovation grant from the Department of Innovation Science and Technology, has played an important role in accelerating the development of core technologies needed in Micro-X’s next-generation products. Tapping into Flinders’ vast expertise and the equipment through Flinders Microscopy and Microanalysis has provided Micro-X with a better understanding of their systems and refined advanced manufacturing processes. Micro-X has a goal to develop, source and manufacture all components from Australia and create jobs in South Australia.

Atomic force microscopy shows SWCNT (left) and SWCNT rings (centre). Pictured right: SEM image of buckyball tubules.
Professor Colin Raston grabbed world headlines in 2015 when he won the Ig Nobel Prize for the development of a ‘Vortex Fluidic Device’ (VFD) that can unboil an egg.
The VFD is a seriously useful invention. It can be used to refold misshapen proteins, produce biodiesel from waste oils, modify the characteristics of wine and facilitate a range of different chemical transformations. Professor Raston’s group is using the VFD to create carbon nanostructures that could increase the efficiency of solar cells and improve polymer composites, sensing devices, electronics and drug delivery.
Dr Kasturi Vimalanathan is working with Professor Raston on these carbon nanostructures, which are made of graphene—single layers of graphite. Single-walled carbon nanotubes (SWCNT) can be cut into specific lengths by using a pulsed laser with various solvents and altered shear forces in the VFD. The VFD can also bend SWCNTs into rings without reactive chemicals or stabilising surfactants. The diameter of the nanorings is controllable—either from 100 to 200 nm or 300 to 700 nm—and production can be readily scaled up.
Dr Vimalanathan has also used the VFD to assemble nanoscale carbon spheres, commonly known as buckyballs (C60), into crystalline nanotubules without stabilising agents and without trapping solvent molecules during crystallisation. The VFD efficiently controls the assembly and can form micrometre-length nanotubules with a hollow diameter of 100-400nm.
During these manipulations, atomic force microscopy and scanning electron microscopy in the Flinders Microscopy and Microanalysis facilities were used to visualise and measure the nanoscale products.
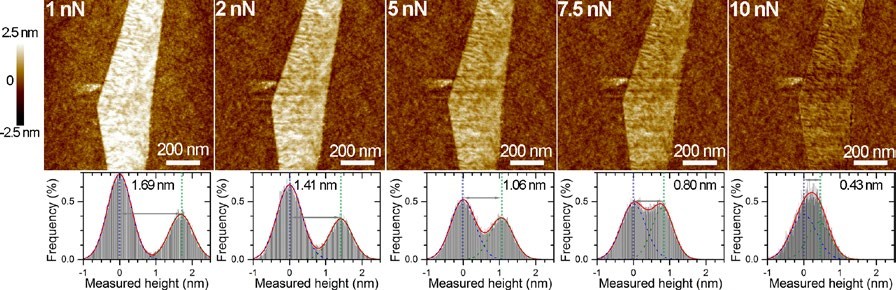
Graphene consists of flat layers of carbon and has emerged as a material with a vast variety of applications. The electronic, optical and mechanical properties of graphene are strongly influenced by the number of layers present in a sample.
As a result, the dimensional characterisation of graphene films is crucial, especially in terms of the thickness and number of layers. Atomic force microscopy (AFM) is often used to determine the thickness of single layer graphene films but a wide range of values from 0.4 to 1.7 nanometres have been reported in the literature.
The major issue with imaging one-atom thick materials is that there is rarely a perfect contact between the substrate and sample. This imperfect contact can be further exacerbated by the presence of a single layer of water molecules, often present on all surfaces under standard conditions. This issue is most commonly observed when imaging with an atomic force microscope (AFM), which directly images a sample in three dimensions using an atomically sharp tip.
Researchers at Flinders University, led by Dr Cameron Shearer and Dr Christopher Gibson, have optimised an AFM technique called PeakForce Tapping AFM to accurately measure graphene by imaging with high force. At low applied force, the measured height is equivalent to the sum of the graphene layer thickness plus that of the intervening water. As the force applied to the graphene by the AFM tip increases, the liquid is gradually pushed out of the way until finally the graphene makes direct contact with the underlying substrate and a much more accurate value is measured. This work was published in the journal, Nanotechnology.

The expertise of Flinders Micrsocopy and Microanalysis, along with researchers from other Micrsocopy Australia facilities and corporate partner, FEI, came together to develop an interactive virtual microscopy experience. Whether you want to view projections on a vaccine nanopatch, surface details of a dinosaur egg or air bubble distribution in frozen ice cream, you can learn to use a scanning electron microscope faster with MyScope. This innovation in training for advanced research offers virtual instruments, step-by-step instructions and expert knowledge. Additional modules covering transmission electron microscopy, x-ray diffraction, scanning probe/atomic force microscopy, light/confocal microscopy, and microanalysis are also available, along with a free Outreach platform designed for ages eight and up.
There is growing demand for high-end education in science and technology across Australia and internationally. Freely available to all, MyScope provides researchers and students with a flexible, individual learning path that can be integrated with traditional learning environments.
'We hope that it will help get more young students excited about entering into science, technology, engineering and math careers,' said John Williams, FEI.
Developed in consultation with science educators in Australia and North America, MyScope Outreach enables discovery at the micro and nanometre scale. Interactive learning is enriched with audio, animations and hands-on activities. Please help us spread the word to promote this innovative STEM resource and bring the thrill of microscopy to Australia’s future scientists.
What we provide
- Expert recommendations
- Prompt and professional service
- Advanced microscopy techniques
- A process and outcomes customised to your needs
- Creative thinking and problem solving
- Training provided by leading instrumentation experts
- Links with government-funded facilities, such as Microscopy Australia and the Australian National Fabrication Facility (ANFF)
What you can expect
- Sample preparation
- Use of advanced microscopy techniques
- Optical and biomedical imaging
- Materials characterisation
- Image processing
- 3D modelling
- Data analysis
The techniques we use
- Metastable induced electron spectroscopy
- Scanning auger nanoprobe
- UPS
- IPES
- XPS
- Atomic force microscopy
- Tip enhanced raman spectroscopy
- Confocal raman microscopy
- Scanning electron microscopy
- X-ray photoelectron spectroscopy
- Neutral impact collision ion scattering spectroscopy
- Confocal fluorescence imaging
Our industry partners work in
- Defence
- Food and agriculture
- Manufacturing
- Maritime
- Technology
CONTACT US
Chat to us about how Flinders Microscopy and Microanalysis can help your business, not-for-profit or government department—or tell us about your research and how you want to use our equipment and laboratories.
MAKE A BOOKING
Relevant contact people and bookings are included in the equipment list.
Our equipment is funded by:



![]()
Sturt Rd, Bedford Park
South Australia 5042
South Australia | Northern Territory
Global | Online


